HIV
Introduction
Since the 1980s, the continuing world-wide AIDS epidemic has killed nearly 33 million [1] people. The efficacy of the Human Immunodeficiency Virus (HIV), the cause of the epidemic, resides in the following:
- it infects the cells that direct the workings of the immune system, eventually suppressing the immune response altogether.
- Its slow-acting nature means that its hosts are not aware of being infected for significant amounts of time, resulting in unwitting transmission via e.g. blood, semen and breastmilk.
- It integrates its own genome into that of the host cell's, thus allowing it to multiply when the cell divides.
The Central Dogma and Reverse Transcription
The central dogma of molecular biology [2] states that genetic information moves in one direction only, from DNA to RNA to protein. However, in the 1970s the dogma had to be restated as “once the genomic information has been translated into protein the process cannot be reversed”, or words to that effect. This modification was warranted by the discovery of reverse transcription, which converts RNA to DNA.
Reverse transcriptase (RT), the enzyme that catalyses reverse transcription, has now been found in all branches of life: bacteria, archaea, eukaryotes and viruses. HIV is one of the viruses that use RT as part of its strategy of replication.
Classification
The Baltimore Classification [3] of HIV is Group VI (+) ssRNA-RT.
-
The ' (+)' denotes that the RNA is positive sense, meaning that it runs in the 5' to 3' direction.
-
'ss' tells us that it is single-stranded.
-
Note that 'RT' here stands for 'retrovirus', i.e. a virus that uses reverse transcription.
Structure
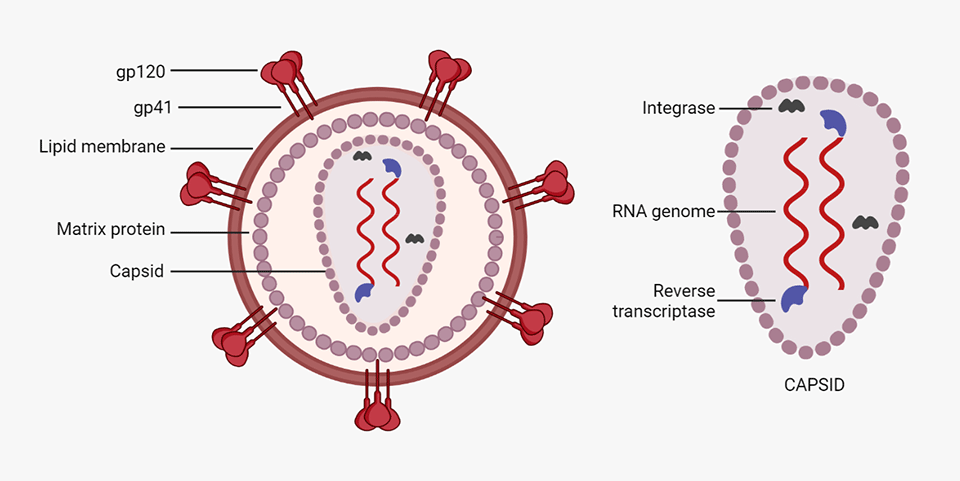
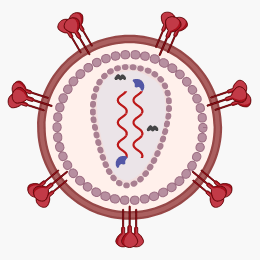
-
Spike proteins are embedded in the lipid membrane, gp120 being the tip of the spike and gp41 the stalk.
-
A matrix protein protects the capsid.
-
The capsid contains:
-
The genomic material, i.e. two [4] strands of ssRNA (+),
-
Reverse transcriptase (RT), which synthesises complementary DNA (cDNA) from the RNA, and
-
Integrase, which incorporates viral cDNA into the host cell's DNA.
-
Replication Cycle
-
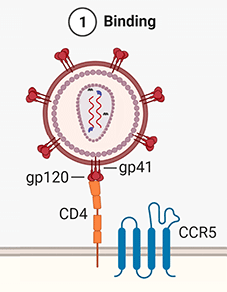
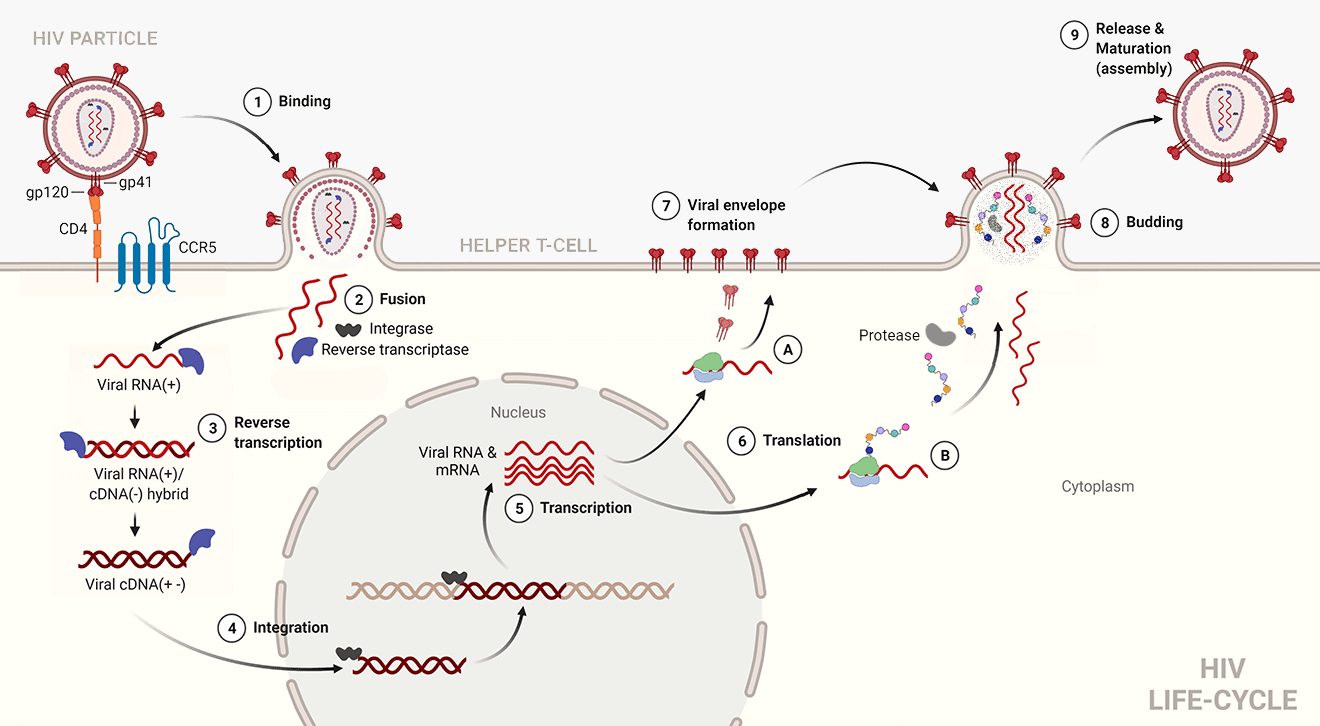
1. Binding. The viral spike protein gp120 binds to the Helper T-cell's CD4 receptor. This causes a conformational change in the CD4 receptor, binding gp120 to the CCR5 co-receptor.
-
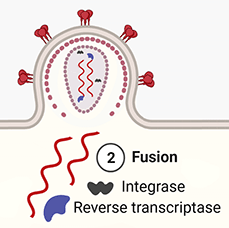
2. Fusion. The binding of gp120 and CCR5 enables the stalk of the spike (gp41) to pierce the T-cell's membrane. This pulls the virus and host membranes together until the virus fuses with the host cell. The inner contents of the virus are then released into the cell, and the matrix and capsid proteins dissolve, releasing the viral RNA, and the RT and integrase enzymes into the cytoplasm.
More detail on binding and fusion
-
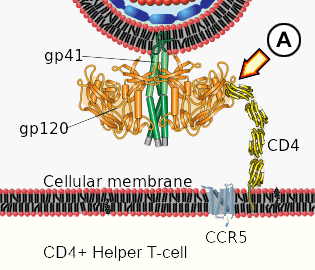
A. Initial interaction between gp120 and CD4 protein.
-
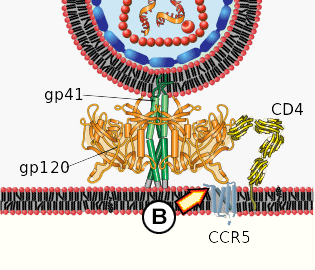
B. Conformational change in gp120 allows for secondary interaction with CCR5.
-
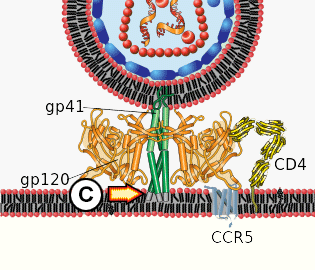
C. The distal tips of gp41 are inserted in to the cellular membrane.
-
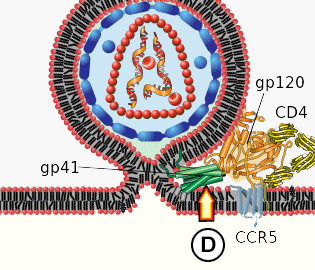
D. gp41 undergoes significant conformational change, folding in half and forming coils. The viral and cellular membranes pull together, fusing them in the process.
-
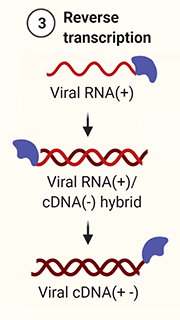
3. Reverse transcription.
-
RT binds to the 3' end of the viral RNA (+).
-
Using dNTPs [5] in the host cell, RT synthesises a complementary strand of cDNA (-). Concurrently, RNase H [6] (see “More detail on reverse transcription”, below), a sub-domain of RT, denatures the RNA strand.
-
Finally, RT synthesises a cDNA (+) strand, resulting in double-sided cDNA (+-).
-
More detail on reverse transcription
RT is an enzyme composed of distinct domains that exhibit different biochemical activities. The predominant functions of RT are RNA-dependent polymerase activity and RNase H activity. However, as is the case with HIV, DNA-dependent DNA polymerase activity is also a possibility. The process of reverse transcription involves the following steps:
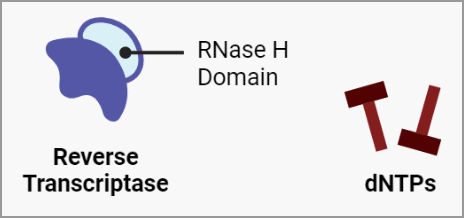
-

A. Viral RNA (+).
-
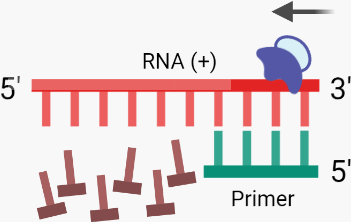
B. Using an RNA fragment as a primer, RT binds to the RNA (+) template, initiating RNA-dependent DNA polymerase activity which synthesises the complementary DNA (cDNA (-)) strand via the incorporation of dNTPs.
-
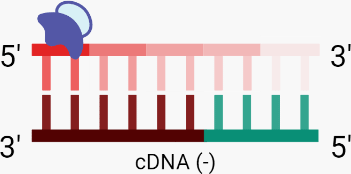
C. Concurrently with cDNA (-) synthesis, RNase H activity denatures (degrades) the RNA template.
-
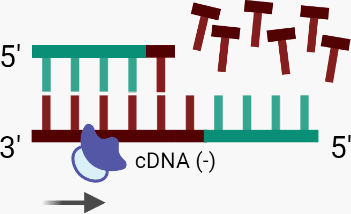
D. Again using an RNA fragment as a primer, DNA-dependent DNA polymerase recognises the single-stranded cDNA (-) as a template and synthesises the second, positive (+) strand of cDNA.
-
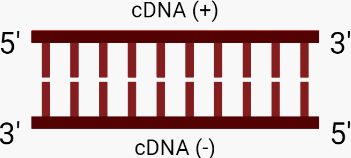
E. The primers are removed, resulting in double-stranded cDNA (+-).
-

4. Integration. The integrase enzyme transports the viral DNA through a pore in the nucleus to the host DNA. Integrase then makes a nick in the host DNA and inserts the viral DNA into it.
-
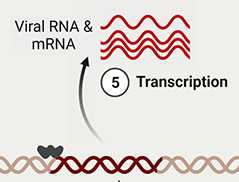
5. Transcription. Host cell RNA polymerase transcribes strands of viral RNA (+), destined for the budding virion (and which are therefore not to be translated) and messenger RNA (mRNA) which will be translated into polyprotein chains. The transcribed RNA exits via nuclear pores into the cytoplasm.
-
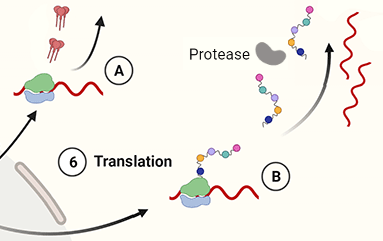
6. Translation. Ribosomes attach to the mRNA strands which are then translated into:
-
A. envelope proteins (gp120 and gp41), which embed themselves in the cell membrane, and
-
B. polyprotein chains, containing the building blocks of the matrix, capsid, RT and integrase enzymes. Protease cleaves itself from the polyprotein chain before entering the virion.
The polyprotein chains, protease, and two strands of viral RNA (+) then move towards the virion which has already begun to bud from the cell envelope.
-
-

7. Viral envelope formation. The viral spike proteins embed themselves in the host cell membrane.
-
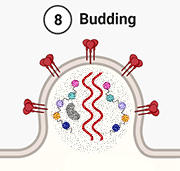
8. Budding. Once all the viral materials have entered the virion, it buds from the host cell.
-
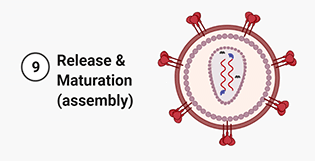
9. Release and Maturation. After budding, the protease cleaves the polypeptide chains into matrix and capsid proteins, integrase and RT. Once these elements have been assembled the virus has reach maturation and is now ready to infect another cell.
The Target of HIV: the Helper T-cell
The virus mainly targets Helper T-cells [7] (also known as Helper T lymphocytes or CD4+ cells). These cells are crucial for the functioning of the adaptive immune system.
Helper T-cells do not attack pathogens directly. Instead, they:
-
bind to T-lymphocytes, stimulating them to differentiate into specialised T-cells, e.g. cytotoxic cells,
-
bind to B-lymphocytes, inducing differentiation into plasma cells, and memory B cells (which produce and store antibodies)
-
release chemicals (cytokines), stimulating defence responses in macrophages, neutrophils, basophils and other cells.
Effect on the T-cell
If the viral DNA embedded in the T-cell genome remains inactivated, generations of mitosis will ensure that it is passed on to generations of daughter cells. Once the viral DNA is activated, large numbers of virions will be produced, and their continual budding will cause the cell to lyse (burst). Other factors contribute to the death of infected T-cells, e.g. apoptosis (programmed cell death) caused by the detection of the viral DNA by specialised host cell proteins.
The result of the death of large numbers of T-cells will be the gradual degradation of the host organism's immune response. As this continues to weaken, the host organism will be rendered ever more vulnerable to a myriad of opportunistic infections which, without treatment, will lead to its death.
AIDS
AIDS stands for Autoimmune Deficiency Syndrome, the umbrella term for the multiple infections that take advantage of the breakdown of the immune system .
While AIDS usually proved fatal in the past, in recent years many drugs have been developed to neutralise the various stages of viral infection. The activities of these drugs include :
-
causing conformational changes in the spike proteins, disallowing the initial docking of the virion with the host cell receptors,
inhibiting RT to stop reverse transcription,
-
inhibiting integrase to stop transport and integration of the viral DNA into the host genome, and
inhibiting the translation of viral DNA into mRNA.
A cocktail of these drugs is often required to successfully treat a sufferer, acting on the principle that if one fails, another will succeed.